Salt-induced reentrant hydrogel of poly(ethylene glycol)–poly(lactide-co-glycolide) block copolymers
Abstract
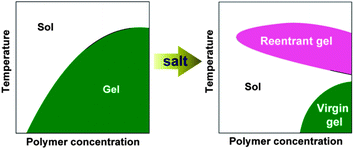
A diblock copolymer of methoxy poly(ethylene glycol) and poly(DL-lactide-co-glycolide) (MPEG–PLGA) was synthesized via ring-opening polymerization. Its aqueous system over the critical gelation concentration underwent a gel-to-sol transition with increasing the temperature. More interestingly, gel-to-sol-to-(reentrant gel) transitions upon heating were found in the presence of salts. The reentrant gel and virgin gel exhibited different viscoelasticities, as revealed by dynamic rheological measurements, and different microstructures displayed in cryo-SEM images. Based on the observations of micelle formation at low copolymer concentrations and NMR detections at high concentrations etc., we anticipated that the virgin gel was formed by the jamming of close-packed micelles and the reentrant gel had, as a thermogel, an internal structure of a percolated micelle network. The phase transition temperatures varied with the salt concentration, and depended also on the type of ion, with the trend obeying the Hofmeister series.

 Please wait while we load your content...
Please wait while we load your content...