Synthesis and characterization of variable conformation pH responsive block co-polymers for nucleic acid delivery and targeted cell entry†
Abstract
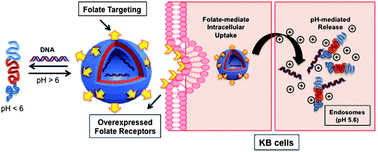
Responsive materials that change conformation with varying pH have been prepared from a range of amphiphilic block co-polymers. The individual blocks are composed of (a) permanently hydrophilic chains with neutral functionality and (b) acrylate polymers with weakly basic side-chains. Variation in co-monomer content, molar mass and block ratios/compositions leads to a range of pH-responses, manifest through reversible self-assembly into micelles and/or polymersomes. These transitions can be tuned to achieve environmental responses in a pH range from 5–7, as shown by turbidimetric analysis, NMR and dynamic light scattering measurements (DLS). Further characterization by transmission electron microscopy (TEM) indicates that polymersomes with diameters of 100–200 nm can be formed under certain pH-ranges where the weakly basic side-chains are deprotonated. The ability of the systems assembled with these polymers to act as pH-responsive containers is shown by DNA encapsulation and release studies, and their potential for application as vehicle for drug delivery is proved by cell metabolic activity and cell uptake measurements.

 Please wait while we load your content...
Please wait while we load your content...