Highly photostable “super”-photoacids for ultrasensitive fluorescence spectroscopy†
Abstract
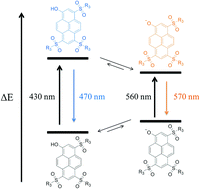
The photoacid 8-hydroxypyren-1,3,6-trisulfonic acid (HPTS, pyranine) is a widely used model compound for the examination of excited state proton transfer (ESPT). We synthesized five “super”-photoacids with varying hydrophilicity and acidity on the basis of HPTS. By chemical modification of the three sulfonic acid substituents, the photoacidity is enhanced by up to more than five logarithmic units from pK*a ≈ 1.4 to ∼−3.9 for the most acidic compound. As a result, nearly quantitative ESPT in DMSO can be observed. The novel photoacids were characterized by steady-state and time-resolved fluorescence techniques showing distinctively red shifted spectra compared to HPTS while maintaining a high quantum yield near 90%. Photostability of the compounds was checked by fluorescence correlation spectroscopy (FCS) and was found to be adequately high for ultrasensitive fluorescence spectroscopy. The described photoacids present a valuable palette for a wide range of applications, especially when the properties of HPTS, i.e. highly charged, low photostability and only moderate excited state acidity, are limiting.

 Please wait while we load your content...
Please wait while we load your content...