Photocatalytic and photoelectrocatalytic degradation of the antibacterial agent ciprofloxacin
Abstract
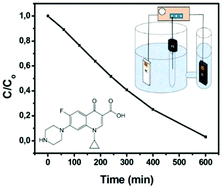
Photocatalytic and photoelectrocatalytic degradation of the antibacterial fluoroquinolone drug, ciprofloxacin, has been studied in the presence of nanocrystalline titania films supported on glass slides or transparent electrodes. The degradation has been examined either in pure water or in the presence of NaOH or NaCl. Titania films can photocatalytically or photoelectrocatalytically degrade ciprofloxacin. In the presence of NaOH, the degradation rate was lower than in pure water and this is explained by the fact that at high pH values attraction of ciprofloxacin to the titania surface is discouraged. In the presence of NaCl, the degradation rate was the highest, thanks to Cl-based radicals which can be photocatalytically created by interacting with photogenerated holes. Application of a forward (anodic) bias increased the photodegradation rate in the presence of both electrolytes while a reverse (cathodic) bias decreased the photodegradation rate. Electrocatalytic effects, i.e. degradation of ciprofloxacin in the dark or in the absence of a photocatalyst under an applied bias of up to ±1.0 V vs. Ag/AgCl, were not detected in the case of NaOH and were of limited importance in the case of NaCl.
- This article is part of the themed collection: Solar Chemistry and Photocatalysis - environmental applications

 Please wait while we load your content...
Please wait while we load your content...