Synthesis of diastereometrically pure cubane-like photodimers from 2,4-pentanediyl-bis-2-naphthoates†‡
Abstract
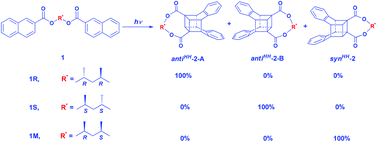
Irradiation of (2R,4R)-(−)- and (2S,4S)-(+)-2,4-pentanediyl-bis-2-naphthoates (1R and 1S, respectively) in organic solutions exclusively results in cubane-like antiHH photodimers in 100% yield. Asymmetric induction with 100% diastereometric excess (de) has been achieved and the absolute configuration of the yielded diastereomers has been established. Moreover, irradiation of (2R,4S)-2,4-pentanediyl-bis-2-naphthoate (1M) gives cubane-like synHH photodimers in 100% yield.
- This article is part of the themed collection: Dedicated to the memory of Prof. Nicholas J. Turro

 Please wait while we load your content...
Please wait while we load your content...