Enantiospecific photochemical 6π-ring closure of α-substituted atropisomeric acrylanilides – role of alkali metal ions†‡
Abstract
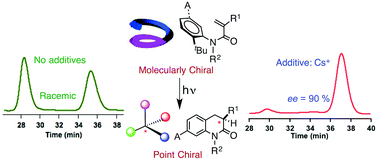
Direct irradiation of atropisomeric α-substituted acrylanilides in the presence of alkali metal ions gave high ee values in the 3,4-dihydro-2-quinolin-2-one photoproduct, while in the absence of alkali metal ions, racemic photoproduct was observed. The heavy atom effect leading to enhanced triplet yields alters the reactive pathway leading to the observed enantioselectivity in the photoproduct.
- This article is part of the themed collection: Dedicated to the memory of Prof. Nicholas J. Turro

 Please wait while we load your content...
Please wait while we load your content...