An indolocarbazole dimer as a new stereodynamic probe for chiral 1,2-diamines†
Abstract
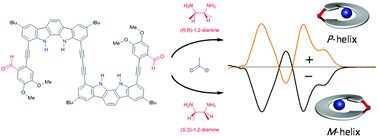
An indolocarbazole dimer that contains aldehyde groups at both ends was prepared by connecting two monomeric units through a rod-like 1,4-butadiynyl spacer. Upon mixing with chiral 1,2-diamines at room temperature, the dimer was in situ converted to the corresponding cyclic diimines in the presence of tetrabutylammonium acetate as a template. The resulting diimines fold to helical conformations of right-handed (P) or left-handed (M) orientations, depending on the absolute stereochemistries of chiral 1,2-diamines. The patterns and intensities of the CD spectra can be used to determine the absolute configurations and enantiomeric excesses of chiral 1,2-diamines.

 Please wait while we load your content...
Please wait while we load your content...