Synthesis of o-chlorophenols via an unexpected nucleophilic chlorination of quinone monoketals mediated by N,N′-dimethylhydrazine dihydrochloride†
Abstract
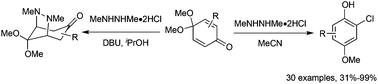
An unexpected nucleophilic chlorination of a quinone monoketal while carrying out a pyrazolidine synthesis has led to a general preparation of multisubstituted phenols. The products are obtained in good to high yields under mild conditions. The bridged pyrazolidines that were the original targets are obtained in the presence of a protic solvent.

 Please wait while we load your content...
Please wait while we load your content...