Asymmetric organocatalytic synthesis of 4,6-bis(1H-indole-3-yl)-piperidine-2 carboxylates†
Abstract
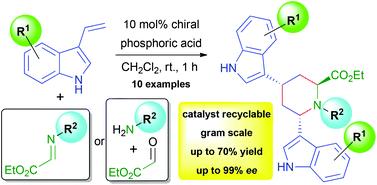
We developed an asymmetric organocatalytic synthesis of 4,6-bis(1H-indole-3-yl)-piperidine-2-carboxylates using 10 mol% of a chiral phosphoric acid. The products, which are novel bisindole-piperidine-amino acid hybrids, can be obtained in one step from 3-vinyl indoles with imino esters in dichloromethane at room temperature after 1 h of reaction time. A variety of these compounds could be synthesized in up to 70% yield and 99% ee, and they were experimentally and computationally analyzed regarding their relative and absolute stereochemistry.

 Please wait while we load your content...
Please wait while we load your content...