Base mediated synthesis of 2-aryl-2,3-dihydroquinazolin-4(1H)-ones from 2-aminobenzonitriles and aromatic aldehydes in water†
Abstract
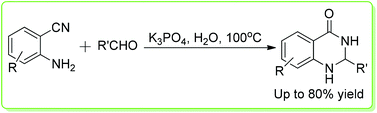
An environmentally friendly and mild procedure to obtain 2,3-dihydroquinazolin-4(1H)-ones from 2-aminobenzonitriles and aromatic aldehydes has been developed. The reactions took place in water with inorganic base (K3PO4) as the only promoter. Various desired products have been prepared in moderate to good yields under identical conditions. Further transformation of dihydroquinazolinones to quinazolinones was carried out as well with TBHP as the oxidant.

 Please wait while we load your content...
Please wait while we load your content...