Contributions of pocket depth and electrostatic interactions to affinity and selectivity of receptors for methylated lysine in water†
Abstract
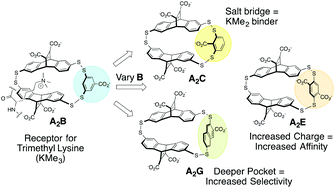
Dynamic combinatorial chemistry was used to generate a set of receptors for peptides containing methylated lysine (KMen, n = 0–3) and study the contribution of electrostatic effects and pocket depth to binding affinity and selectivity. We found that changing the location of a carboxylate resulted in an increase in preference for KMe2, presumably based on ability to form a salt bridge with KMe2. The number of charged groups on either the receptor or peptide guest systematically varied the binding affinities to all guests by approximately 1–1.5 kcal mol−1, with little influence on selectivity. Lastly, formation of a deeper pocket led to both increased affinity and selectivity for KMe3 over the lower methylation states. From these studies, we identified that the tightest binder was a receptor with greater net charge, with a Kd of 0.2 μM, and the receptor with the highest selectivity was the one with the deepest pocket, providing 14-fold selectivity between KMe3 and KMe2 and a Kd for KMe3 of 0.3 μM. This work provides key insights into approaches to improve binding affinity and selectivity in water, while also demonstrating the versatility of dynamic combinatorial chemistry for rapidly exploring the impact of subtle changes in receptor functionality on molecular recognition in water.
- This article is part of the themed collection: Supramolecular Chemistry in Water

 Please wait while we load your content...
Please wait while we load your content...