Oxidative activation of dihydropyridine amides to reactive acyl donors†
Abstract
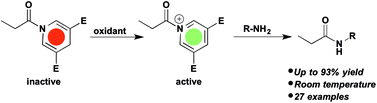
Amides of 1,4-dihydropyridine (DHP) are activated by oxidation for acyl transfer to amines, alcohols and thiols. In the reduced form the DHP amide is stable towards reaction with amines at room temperature. However, upon oxidation with DDQ the acyl donor is activated via a proposed pyridinium intermediate. The activated intermediate reacts with various nucleophiles to give amides, esters, and thio-esters in moderate to high yields.

 Please wait while we load your content...
Please wait while we load your content...