Decarboxylative allylations of ester enolate equivalents†
Abstract
A variety of ester enolate equivalents are generated in situ and undergo α-allylation in high yields via palladium-catalyzed decarboxylative allylation. The transformations are complete within very short reaction times under ambient conditions. Synthesis of α-allylated acyl derivatives provides access to other carboxylic acid and alcohol derivatives via acyl group substitution or reduction.
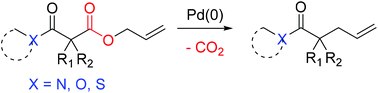

 Please wait while we load your content...
Please wait while we load your content...