Direct C–H bond arylation of fluorenes with aryl chlorides catalyzed by N-heterocyclic carbene–palladium(ii)–1-methylimidazole complex and further transformation of the products in a one-pot procedure†
Abstract
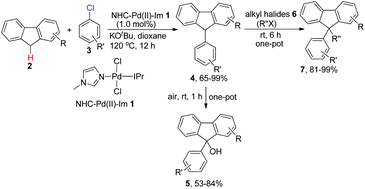
We report here the NHC–Pd(II)–Im complex 1-catalyzed direct C–H bond functionalization of the C9 position of fluorenes with aryl chlorides and further transformation of the resulting products in a one-pot procedure. Under the optimal conditions, arylated fluorenes can be obtained in moderate to almost quantitative yields using various activated and unactivated (hetero)aryl chlorides as the arylating reagents. Furthermore, if the mixture from the arylation reaction is exposed to air, the C9-oxidized products can be obtained in acceptable to good yields in a one-pot procedure. In addition, alkyl groups can also be efficiently introduced to the above mixture from the arylation reaction, producing further C9-alkylated products in good to almost quantitative yields in a one-pot procedure, thus providing an expedient, inexpensive and practical strategy for the mono- and di-functionalization of fluorenes.

 Please wait while we load your content...
Please wait while we load your content...