Clicked bis-PEG-peptide conjugates for studying calmodulin-Kv7.2 channel binding†
Abstract
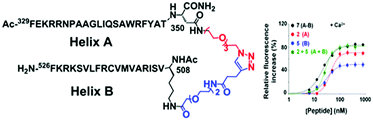
The recombinant Kv7.2 calmodulin (CaM) binding site (Q2AB CaMBD) shows a high tendency to aggregate, thus complicating biochemical and structural studies. To facilitate these studies we have conceived bis-PEG-peptide CaMBD-mimetics linking helices A and B in single, easy to handle molecules. Short PEG chains were selected as spacers between the two peptide molecules, and a Cu(I)-catalyzed cycloaddition (CuAAC) protocol was used to assemble the final bis-PEG-peptide conjugate, by the convenient functionalization of PEG arms with azide and alkyne groups. The resulting conjugates, with a certain helical character in TFE solutions (CD), showed nanomolar affinity in a fluorescence CaM binding in vitro assay, higher than just the sum of the precursor PEG-peptide affinities, thus validating our design. The approach to these first described examples of Kv7.2 CaMBD-mimetics could pave the way to chimeric conjugates merging helices A and B from different Kv7 subunits.

 Please wait while we load your content...
Please wait while we load your content...