Neither azeotropic drying, nor base nor other additives: a minimalist approach to 18F-labeling†
Abstract
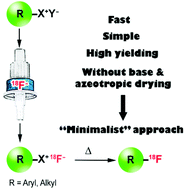
A novel, efficient, time-saving and reliable radiolabeling procedure via nucleophilic substitution with [18F]fluoride is described. Different radiolabeled aliphatic and aromatic compounds were prepared in high radiochemical yields simply by heating of quaternary anilinium, diaryliodonium and triarylsulfonium [18F]fluorides in suitable solvents. The latter were obtained via direct elution of 18F− from an anion exchange resin with alcoholic solutions of onium precursors. Neither azeotropic evaporation of water, nor a base, nor any other additives like cryptands or crown ethers were necessary. Due to its simplicity this method should be highly suitable for automated radiosyntheses, especially in microfluidic devices.

 Please wait while we load your content...
Please wait while we load your content...