Palladium-catalyzed one pot 2-arylquinazoline formation via hydrogen-transfer strategy†
Abstract
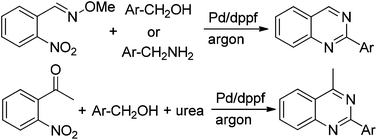
The palladium catalytic system was first applied to 2-arylquinazoline synthesis via hydrogen transfer methodology. Various (E)-2-nitrobenzaldehyde O-methyl oximes reacted easily with alcohols or benzyl amines to provide N-heterocyclic compounds in good to high yields. Similarly, the heterocyclic products could be prepared by the reaction of 1-(2-nitrophenyl)ethanone, urea and benzyl alcohols. In these reactions, the nitro group was reduced in situ by hydrogen generated from the alcohol dehydrogenation step.

 Please wait while we load your content...
Please wait while we load your content...