A facile manganese dioxide mediated oxidation of primary benzylamines to benzamides†
Abstract
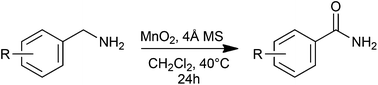
A high yielding manganese dioxide mediated oxidation of benzylamines to the corresponding amides has been developed under mild reaction conditions. The mechanism for the conversion has been explored by 1H NMR spectroscopy and the role of both manganese dioxide and molecular sieves in the reaction elucidated.

 Please wait while we load your content...
Please wait while we load your content...