Asymmetric borylation of α,β-unsaturated esters catalyzed by novel ring expanded N-heterocyclic carbenes based on chiral 3,4-dihydro-quinazolinium compounds†
Abstract
A series of chiral 6-membered ring N-heterocyclic carbene (NHC) precursors based on 3,4-dihydro-quinazoline were synthesized with overall yields of 54–62%. NHCs generated from these precursors show excellent asymmetric catalytic properties for borylation of α,β-unsaturated esters with enantioselectivity of up to 93% with a catalyst content of only 1 mol%.
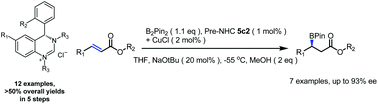

 Please wait while we load your content...
Please wait while we load your content...