An easy access to α-aryl substituted γ-ketophosphonates: Lewis acid mediated reactions of 1,3-diketones with α-hydroxyphosphonates and tandem regioselective C–C bond cleavage†
Abstract
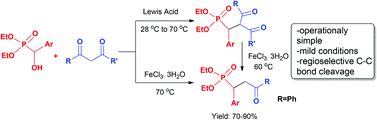
A range of α-aryl substituted γ-ketophosphonates is synthesised by Lewis acid mediated reactions of 1,3-diketones and easily accessible, inexpensive benzylic α-hydroxyphosphonates in an operationally simple method under solvent-free conditions without exclusion of air/moisture. A regioselective C–C bond cleavage for 1,3-diketones in a tandem fashion has also been demonstrated. Synthesis of a γ-ketophosphonate with phenol functionality at the α-position (structural analogue of raspberry ketone, a natural product) has also been presented.

 Please wait while we load your content...
Please wait while we load your content...