Stereoselective one-pot synthesis of β-alkylsulfide enol esters. Base-triggered rearrangement under mild conditions†
Abstract
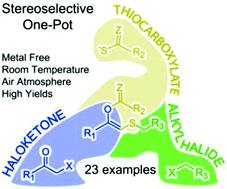
A stereoselective one-pot procedure was developed to prepare S-substituted (Z)-enol esters through a base-triggered rearrangement. This transition metal-free multicomponent approach can be performed under an air atmosphere at room temperature, tolerates a wide set of chemical functionalities and generally affords high isolated yields. The (Z)-selectivity arises from the [1,4]-S- to O-acyl migration.

 Please wait while we load your content...
Please wait while we load your content...