Catalytic asymmetric nucleophilic openings of 3-substituted oxetanes
Abstract
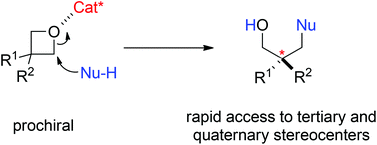
Asymmetric ring-opening of 3-substituted oxetanes provides rapid access to highly functionalized chiral building blocks. However, progress in this field is limited. Recently we developed a new catalytic system based on chiral Brønsted acids for this type of reaction and demonstrated the synthesis of a range of useful molecules under mild and operationally simple conditions. In this perspective, we describe the challenges, progress, and potential future efforts on this topic.

 Please wait while we load your content...
Please wait while we load your content...