A new synthetic approach to 6-unsubstituted phenanthridine and phenanthridine-like compounds under mild and metal-free conditions†
Abstract
A new and mild synthetic approach for the synthesis of 6-unsubstituted phenanthridine and phenanthridine-like compounds under metal-free conditions at room temperature has been developed. The strategy involved a tandem azide rearrangement/intramolecular annulation and oxidation reactions of biarylmethyl azide precursors to obtain the desired products in up to 99% yields with high regioselectivity.
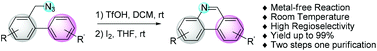

 Please wait while we load your content...
Please wait while we load your content...