A succinyl lysine-based photo-cross-linking peptide probe for Sirtuin 5†
Abstract
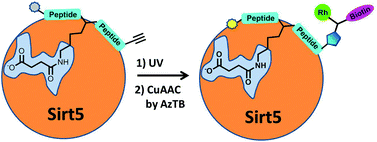
A succinylation-specific photo-cross-linking peptide probe has been developed for the NAD+-dependent hydrolase Sirtuin 5. The probe, not only displayed robust labelling performance with purified Sirt5, but also enabled sensitive detection of the hydrolase in the presence of large excess of cellular proteins. It is anticipated that this probe, and future generations of it, will provide useful chemical tools for the functional analysis of Sirt5 and for the recently discovered PTM of lysine succinylation.

 Please wait while we load your content...
Please wait while we load your content...