Rhodium(iii)-catalyzed regioselective C2-amidation of indoles with N-(2,4,6-trichlorobenzoyloxy)amides and its synthetic application to the development of a novel potential PPARγ modulator†
Abstract
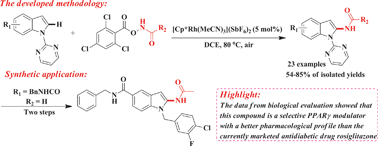
A new and efficient method for the direct regioselective C2-amidation of various functionalized indoles with several N-(2,4,6-trichlorobenzoyloxy)amides via Rh(III)-catalyzed C–H activation/N–O cleavage/C–N formation using the pyrimidyl group as a readily installable and removable directing group has been developed. With this method, a variety of valuable 2-amido indoles can be easily prepared under mild conditions with broad functional group tolerance and excellent region-/site-specificities. Application of this strategy to the synthesis of target compound 6 as a novel PPARγ modulator was also demonstrated. The results from biological evaluation showed that compound 6 had a partial PPARγ agonistic activity and a strong PPARγ binding affinity with an IC50 value of 120.0 nM, along with a less pronounced adipocyte differentiation ability compared to the currently marketed anti-diabetic drug rosiglitazone, suggesting that further development of such a compound might be of great interest.

 Please wait while we load your content...
Please wait while we load your content...