CuI-catalyzed cross-coupling of terminal alkynes with dialkoxycarbenes: a general method for the synthesis of unsymmetrical propargylic acetals†
Abstract
A general source of dialkoxycarbenes, 2,2-dialkoxy-5,5-dimethyl-Δ3-1,3,4-oxadiazolines, have been successfully employed as coupling partners in CuI-catalyzed cross-coupling reactions with terminal alkynes, which afforded various unsymmetrical propargylic acetals in good yields.
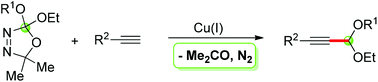

 Please wait while we load your content...
Please wait while we load your content...