A chemoselective oxidation of monosubstituted ethylene glycol: facile synthesis of optically active α-hydroxy acids†
Abstract
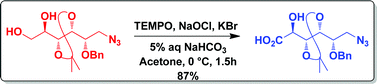
A mild and efficient method for the synthesis of optically active α-hydroxy acids through chemoselective oxidation of monosubstituted ethylene glycols using the TEMPO–NaOCl reagent system is described. It is evident from our studies that the solvent, pH and reaction temperature are very crucial for the success of this oxidation. The versatility of this method has been demonstrated with a variety of aliphatic, aromatic and carbohydrate substrates bearing various functional groups.

 Please wait while we load your content...
Please wait while we load your content...