Organocatalytic enantioselective construction of multi-functionalized spiro oxindole dienes†
Abstract
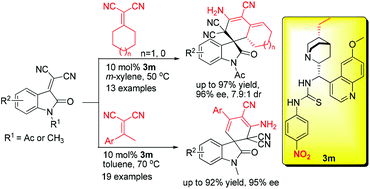
A diastereo- and enantio-selective domino Michael-cyclization–tautomerization reaction of isatylidene malononitriles with α,α-dicyanoalkenes catalyzed by a cinchona alkaloid-derived bifunctional thiourea catalyst has been developed. A series of multi-functionalized spiro oxindole diene derivatives have been obtained in good to excellent yields (up to 97%) with good to excellent enantioselectivities (up to 96%) as well as good diastereoselectivities (up to 7.9 : 1). In addition, an anomalous temperature effect on the enantioselectivity has also been studied for this transformation.

 Please wait while we load your content...
Please wait while we load your content...