Effect of C7-substitution of 1-arylsulfonyl-5-(N-hydroxyacrylamide)indolines on the selectivity towards a subclass of histone deacetylases†
Abstract
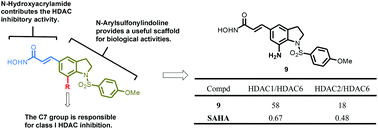
This study focused on the substitution effect at position C7 of 1-arylsulfonyl-5-(N-hydroxyacrylamide)indolines. Compound 9, (E)-3-(7-amino-1-(4-methoxyphenylsulfonyl)indolin-5-yl)-N-hydroxyacrylamide, displayed 4- to 14-fold more potent antiproliferative activity than vorinostat (SAHA, 1). Notably, 9 possessed specific histone deacetylase (HDAC) inhibitory activity toward HDAC1 and HDAC2, but had no effect on HDAC6, indicating that 9 has the potential to be developed as a class I HDAC inhibitor. In a xenograft tumor model, 9 suppressed the growth of HCT116 cells at 100 mg kg−1, which led to a TGI (tumor growth inhibition) of 40.3%. Taken together, the C7 substitutions have a crucial effect on class I HDACs, which is beneficial for synthesizing efficient anticancer agents.

 Please wait while we load your content...
Please wait while we load your content...