Evaluation of 4-substituted styrenes as functional monomers for the synthesis of theophylline-specific molecularly imprinted polymers†
Abstract
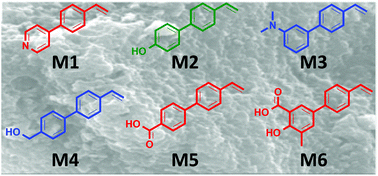
Six novel functional monomers: 4-(4-vinylphenyl)pyridine (M1), 4′-vinylbiphenyl-4-ol (M2), N,N-dimethyl-4′-vinylbiphenyl-3-amine (M3), (4′-vinylbiphenyl-4-yl)methanol (M4), 4′-vinylbiphenyl-4-carboxylic acid (M5) and 4-hydroxy-5-methyl-4′-vinylbiphenyl-3-carboxylic acid (M6), were examined for their ability to imprint theophylline (1). Using a molecular modelling-NMR titration approach, M2 and M6 were predicted to give rise to the most specific molecularly imprinted polymers (MIPs). Rebinding analysis suggests that no imprinting effect resulted from the polymerisation of monomers M1, M5 and M6, but modest to good levels of imprinting were evident from monomers M2, M3 and M4 with IF values ranging from 1.1 (MIPM3, 20 mg) to 45 (MIPM2, 10 mg). The selective recognition of 1 varied as a function of polymer mass used. At low polymer loadings MIPM2 gave the very high IF of 45, reducing to IF = 4.1–2.3 at 20–40 mg polymer loading. With monomer M2, microwave synthesised MIP (MW-MIPM2) was examined. The MW-MIPM2 displayed lower specific rebinding than its conventionally produced counterpart (MIPM2) with IF values ranging from 1.6–2.3 (cf., MIPM2 IF 2.3–45), but significantly higher levels of rebinding with 25–52% of 1 rebound from a 0.080 mM CH3CN solution of 1 (cf., MIPM2 5–25%). MW-MIPM2 displayed a lower BET surface area than MIPM2 (185 m2 g−1vs. 240 m2 g−1), and lower surface (zeta) potential (−13.1 ± 8.22 mV vs. −31.4 ± 4.84 mV). Freundlich isotherm analysis revealed that MW-MIPM2 possessed higher affinity binding sites for 1 than MIPM2 with Kd values of 1.38 and 2.31 respectively. In addition, MW-MIPM2 also exhibits a higher number of binding sites (NT) compared to MW-NIPM2 (0.72 and 0.41 mg g−1, respectively). In specificity studies using caffeine (2), MIPM2 displayed a two-fold preference for rebinding of 1 and MW-MIPM2 a five-fold preference for 1 over 2. The quantity of 2 bound in both cases was consistent with non-specific binding events. In competitive rebinding experiments, increased discrimination in favour of 1 over 2 was observed.

 Please wait while we load your content...
Please wait while we load your content...