Lewis acid promoted dual bond formation: facile synthesis of dihydrocoumarins and spiro-tetracyclic dihydrocoumarins†
Abstract
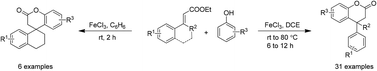
Lewis acid (FeCl3) mediated dual bond (C–C and C–O) formation for synthesis of 3,4-dihydrocoumarins is presented. This method has successfully delivered a number of dihydrocoumarins containing dense functionalities on the aromatic ring. Significantly, the present method enabled achieving dihydrocoumarins with tertiary as well as quaternary carbon atoms at the benzylic position. Gratifyingly, the novel spiro-tetracyclic lactones have also been dextrously prepared using this process.

 Please wait while we load your content...
Please wait while we load your content...