Design, synthesis and biological evaluation of bicyclic iminosugar hybrids: conformational constraint as an effective tool for tailoring the selectivity of α-glucosidase inhibitors†
Abstract
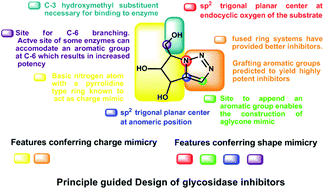
Principle guided design of glycan processing enzyme inhibitors involves embedding aromatic groups onto charge and shape mimics. Intramolecular azide–alkyne cycloaddition was used as a simple and versatile strategy for the synthesis of novel condensed bicyclic triazoles from carbohydrate derived Perlin aldehydes. These newly synthesised molecules were evaluated for glycosidase inhibition against 11 commercially available enzymes and were found to possess significant affinity (micromolar range) as well as high degree of selectivity for α-glucosidases. Conformational restriction was identified as an important tool to customize the selectivity of enzyme inhibition by five-membered iminosugars.

 Please wait while we load your content...
Please wait while we load your content...