A stereoselective approach to indolizidine and pyrrolizidine alkaloids: total synthesis of (−)-lentiginosine, (−)-epi-lentiginosine and (−)-dihydroxypyrrolizidine†
Abstract
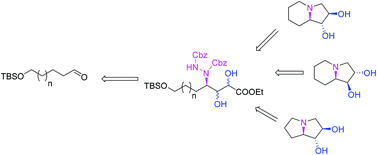
A simple and highly efficient approach to hydroxylated pyrrolizidine and indolizidine is developed from an aldehyde as a starting material using organocatalytic and asymmetric dihydroxylation reactions as key steps. Its application to the total synthesis of (−)-lentiginosine, (−)-epi-1,2-lentiginosine and (−)-dihydroxypyrrolizidine is also reported.

 Please wait while we load your content...
Please wait while we load your content...