Diastereoselective formation of aziridines from diazocarbonyl compounds and N-(O-pivaloylated d-galactosyl)benzylideneamines and ring-opening reactions with p-toluenethiol†
Abstract
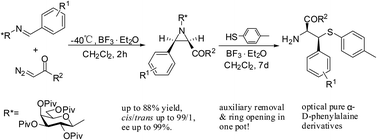
N-Galactosyl aziridines were synthesized via BF3·OEt2 promoted addition of carbenes generated from diazocarbonyl compounds with O-pivaloylated β-D-galactosylimines in good yields and high diastereoselectivity. The ring-opening reactions with p-toluenethiol of the aziridines provided enantiometrically pure β-S-substituted phenylalanine derivatives in a highly regioselective manner.

 Please wait while we load your content...
Please wait while we load your content...