Development and application of serine/threonine ligation for synthetic protein chemistry
Abstract
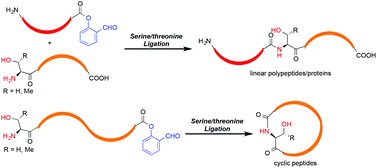
Chemical synthesis of proteins, especially those with post-translational modifications, has offered new opportunities to study the protein structure–function relationship. In the past four years, we have developed the serine/threonine ligation (STL), which involves the chemoselective reaction between peptide salicylaldehyde esters and peptides with N-terminal serine or threonine. The method has been successfully applied to the synthesis of both linear and cyclic peptides/proteins.

 Please wait while we load your content...
Please wait while we load your content...