Design and synthesis of lipid-coupled inositol 1,2,3,4,5,6-hexakisphosphate derivatives exhibiting high-affinity binding for the HIV-1 MA domain†
Abstract
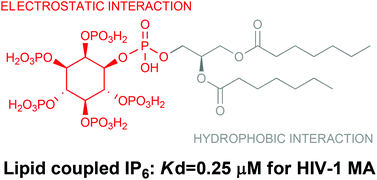
The precursor of Gag protein (Pr55Gag) of human immunodeficiency virus, the principal structural component required for virus assembly, is known to bind D-myo-phosphatidylinositol 4,5-bisphosphate (PIP2). The N-terminus of Pr55Gag, the MA domain, plays a critical role in the binding of Pr55Gag to the plasma membrane. Herein, we designed and synthesized myo-phosphatidylinositol 2,3,4,5,6-pentakisphosphate (PIP5) derivatives comprising highly phosphorylated inositol and variously modified diacylglycerol to examine the MA-binding properties. The inositol moiety was synthesized starting with myo-inositol and assembled with a hydrophobic glycerol moiety through a phosphate linkage. The Kd value for MA-binding of the PIP5 derivative 2 (Kd = 0.25 μM) was the lowest (i.e., highest affinity) of all derivatives, i.e., 70-fold lower than the Kd for the PIP2 derivative 1 (Kd = 16.9 μM) and 100-fold lower than the Kd for IP6 (Kd = 25.7 μM), suggesting the possibility that the PIP5 derivative blocks Pr55Gag membrane binding by competing with PIP2 in MA-binding.

 Please wait while we load your content...
Please wait while we load your content...