Conformational analysis of helical aminoisobutyric acid (Aib) oligomers bearing C-terminal ester Schellman motifs†
Abstract
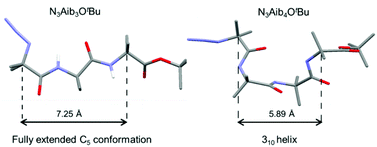
The effect of Schellman motifs on the adoption of stable 310 helical conformations in a series of aminoisobutyric (Aib) oligomers has been studied in the solid state and solution. The destabilising effect of the Schellman motif (a local inversion of helical screw-sense due to a C-terminal ester residue) was quantified in the solid state using X-ray crystallography through analysis of the torsion angles and their deviation from those observed in an ideal 310 helix. Investigation of the intramolecular hydrogen-bonding interactions in the solid state led to the identification of a fully extended C5 conformation in one oligomer, which is a novel folding motif for Aib oligomers. The effect of ester groups with differing steric demands on intermolecular hydrogen-bonding contacts in the solid state was also ascertained. In solution, the adoption of a 310 conformation in Aib oligomers appeared to be more finely tuned, depending on a number of factors, including chain length and the steric demands of the C-terminal destabilising Schellman motif.

 Please wait while we load your content...
Please wait while we load your content...