A concise approach for the synthesis of bitungolides: total syntheses of (−)-bitungolide B & E†
Abstract
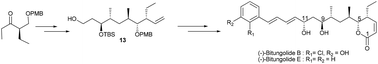
The first total synthesis of (−)-bitungolide B and a second-generation total synthesis of (−)-bitungolide E are described. The cornerstone of the approach comprises a convergent and flexible route involving Brown crotylation, highly diastereoselective substrate controlled Paterson anti-aldol reaction, hydroxyl-directed 1,3-syn/anti reduction, Barton–McCombie deoxygenation and RCM reactions. Via this route, a common intermediate 13 is readily accessible for the synthesis of the family of bitungolides A–E and franklinolides A–C.

 Please wait while we load your content...
Please wait while we load your content...