Copper-catalyzed benzylic C(sp3)–H alkoxylation of heterocyclic compounds†
Abstract
We achieved intra- and intermolecular C(sp3)–H alkoxylation of benzylic positions of heteroaromatic compounds using CuBrn (n = 1, 2)/5,6-dimethylphenanthroline (or 4,7-dimethoxyphenanthroline) and (tBuO)2 as a catalyst and an oxidant, respectively. The reaction proceeded at both terminal and internal benzylic positions of the alkyl groups. The intramolecular alkoxylation was performed on a gram scale.
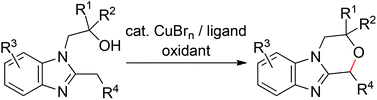

 Please wait while we load your content...
Please wait while we load your content...