“Click and go”: simple and fast folic acid conjugation†
Abstract
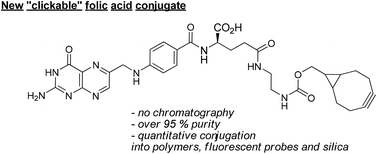
Folic acid targeting by functionalization of the terminal γ-carboxylic acid is one of the most important strategies to selectively deliver chemotherapeutics and dyes to cancer cells which overexpress folate receptors. However, conjugation of folic acid is limited by its unique solubility and by selectivity issues imposing the need for expensive preparative reverse-phase chromatographic purification to isolate γ-folate conjugates. Herein is provided a novel synthetic tool for the synthesis of new folic acid conjugates with excellent γ-purity based on strain-promoted alkyne–azide cycloadditions with a γ-folate–cyclooctyne conjugate 3. To demonstrate the potential of this methodology several new folate conjugates were synthesized with high γ-purity and without using any type of chromatographic purification by reacting conjugate 3 with several fluorescent probes, polymers and siliceous materials bearing azide. In addition, the cycloaddition reaction between conjugate 3 and an azido-derived fluorescent dye was successfully performed in cellular media leading to an increase of fluorescence in the cells which overexpress folate receptors (NCI-H460).

 Please wait while we load your content...
Please wait while we load your content...