Tetrathiafulvalene mono- and bis-1,2,3-triazole precursors by click chemistry: structural diversity and reactivity†
Abstract
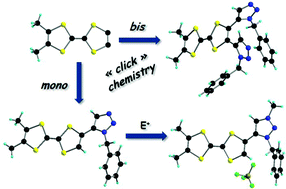
The donor ortho-dimethyl-TTF-(N-n-Bu-1,2,3-triazole) 1,5-isomer has been synthesized by click chemistry following a ruthenium-catalyzed azide–alkyne cycloaddition procedure. The single crystal X-ray analysis showed a planar conformation between the TTF and triazole units and a set of intermolecular interactions at the supramolecular level in the solid state. The same procedure allowed the preparation of the corresponding ortho-dimethyl-TTF-bis(triazole) which was also structurally characterized. Because of the steric hindrance, the triazole units are no longer planar with the TTF backbone. The reactivity of the triazole ring has been investigated in protonation and alkylation reactions, monitored by UV-visible spectroscopy, which clearly showed the red shift of the intramolecular charge transfer band. A TTF-methyl-triazolium salt has been isolated and analyzed by single crystal X-ray analysis. All of the TTF-triazoles and triazolium salts are valuable precursors for radical cation salts due to their oxidation potentials and variety of possible intermolecular interactions.

 Please wait while we load your content...
Please wait while we load your content...