Regioselective 1,4- over 1,2-addition of 3,3-bis(silyl) allyloxy lithium to enals, enones and enoates. The remarkable α-effect of silicon†
Abstract
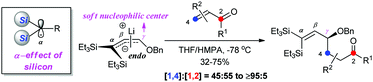
A remarkable α-effect of silicon has been discovered that results in soft nucleophilicity at the Cγ of 3,3-bis(silyl) allyloxy lithium 1. The addition of 1 to α,β-unsaturated carbonyl compounds, including enals, proceeds in a 1,4- over 1,2-manner with medium to good regioselectivity, whereas the parent allyloxy lithium 4 undergoes complete 1,2-addition. The results from DFT calculations of HMPA-complexed 1 and 4 provide the rationale to explain this different regioselectivity.

 Please wait while we load your content...
Please wait while we load your content...