Tandem halogenation/Michael-initiated ring-closing reaction of α,β-unsaturated nitriles and activated methylene compounds: one-pot diastereoselective synthesis of functionalized cyclopropanes†
Abstract
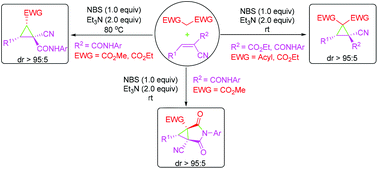
An efficient one-pot synthetic route to highly substituted cyclopropanes has been developed from readily available α,β-unsaturated nitriles and doubly activated methylene compounds under very mild conditions in a highly diastereoselective manner, which involves halogenation, Michael addition and intramolecular ring-closing reaction sequences.

 Please wait while we load your content...
Please wait while we load your content...