RNA–peptide conjugate synthesis by inverse-electron demand Diels–Alder reaction†
Abstract
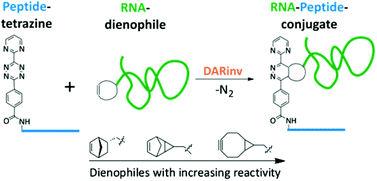
Here we report an efficient method for the synthesis of RNA–peptide conjugates by inverse-electron demand Diels–Alder reaction. Various dienophiles were enzymatically incorporated into RNA and reacted with a chemically synthesized diene-modified peptide. The Diels–Alder reaction proceeds with near-quantitative yields in aqueous solution with stoichiometric amounts of reactants, even at low micromolar concentrations.
 Please wait while we load your content...
Please wait while we load your content...