Structure-based de novo design and identification of D816V mutant-selective c-KIT inhibitors†
Abstract
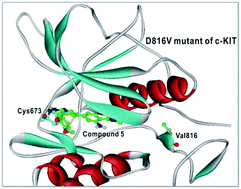
To identify potent and selective inhibitors of D816V, the most common gain-of-function c-KIT mutant, we carried out structure-based de novo design using 7-azaindole as the core and the scoring function improved by implementing an accurate solvation free energy term. This approach led to the identification of new c-KIT inhibitors specific for the D816V mutant. The 3-(3,4-dimethoxyphenyl)-7-azaindole scaffold was optimized and represents a lead structure for the design of the potent and specific inhibitors of the D816V mutant. The results of molecular dynamics simulations indicate that hydrogen bonding interactions between the 7-azadindole moiety and the backbone groups of Cys673 are the most significant determinant for the potency and selectivity of c-KIT inhibitors.
 Please wait while we load your content...
Please wait while we load your content...