Trapping the Lewis acid generated transient species from pentafulvene derived diazanorbornenes with ortho-functionalized aryl iodides and aliphatic alcohols†
Abstract
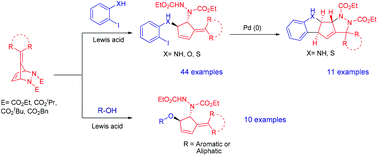
Herein we describe our efforts on the Lewis acid catalyzed stereoselective ring-opening of pentafulvene derived diazabicyclic olefins using various ortho-functionalized aryl iodides such as 2-iodoanilines, 2-iodophenols and 2-iodobenzene thiols to access trans-1,2 disubstituted alkylidenecyclopentenes. The scope of the reaction was also explored with a range of easily available aromatic and aliphatic alcohols. Furthermore, the palladium catalyzed intramolecular Heck cyclization of trans-1,2 disubstituted alkylidenecyclopentenes would provide an easy approach for the synthesis of highly functionalized spiropentacyclic frameworks consisting of a cyclopentene fused to an indoline/benzothiophene and pyrazolidine.

 Please wait while we load your content...
Please wait while we load your content...