Unusual truncation of N-acylated peptoids under acidic conditions†
Abstract
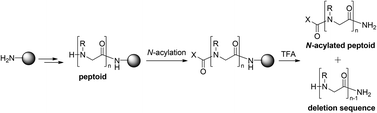
The terminal amino groups of peptoids have often been protected with acetyl groups to improve cell permeability and therapeutic potential, and to prevent the poisoning of the catalysts in organometallic reactions. Interestingly, the unusual truncation of the terminal peptoid unit has sometimes been encountered when the acetylated linear peptoids were treated with a TFA cleavage cocktail. In this study, we systematically investigated the electronic effects of acyl groups on the truncation of N-acylated peptoids to rationalize the formation of the deleted peptoids and to establish an appropriate strategy for preventing such undesired truncation.

 Please wait while we load your content...
Please wait while we load your content...