Synthesis of carbazole-based hetero-core-modified porphyrins†
Abstract
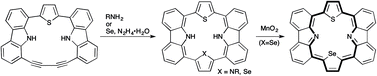
Cu(I)-mediated annulation reaction of a 1,1′-(1,3-butadiyne)-8,8′-(2,5-thiophene)-bridged carbazole dimer 10 with amines provided the N-substituted carbazole-based isophlorines 11a–11c. A similar annulation reaction with selenium in the presence of hydrazine monohydrate afforded hetero-core-modified isophlorine 12. The oxidation of 12 generated the corresponding 21-selena-23-thiaporphyrin 13, which exhibited NIR absorption. The intramolecular charge transfer from Se to S was confirmed by the 1H NMR results along with DFT calculations.

 Please wait while we load your content...
Please wait while we load your content...