A highly efficient catalyst of a nitrogen-based ligand for the Suzuki coupling reaction at room temperature under air in neat water†
Abstract
Glycine, as a kind of commercially available and inexpensive ligand, is used to prepare an air-stable and water-soluble catalyst for the Suzuki–Miyaura reaction in our study. In the presence of 0.1% [PdCl2(NH2CH2COOH)2] as the catalyst, extremely excellent catalytic activity towards the Suzuki–Miyaura coupling of aryl halides containing the carboxyl group with various aryl boronic acids is observed at room temperature under air in neat water.
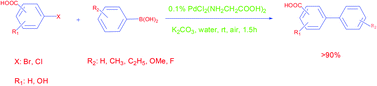

 Please wait while we load your content...
Please wait while we load your content...