Iridium-catalyzed ortho-C–H borylation of aromatic aldimines derived from pentafluoroaniline with bis(pinacolate)diboron†
Abstract
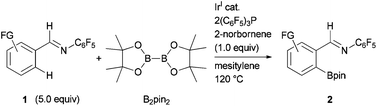
The development of an Ir-catalyzed ortho-C–H borylation of aromatic aldimines derived from pentafluoroaniline is reported. This reaction proceeded at 120 °C to afford the corresponding borylated products in high yield with good regioselectivity using an Ir complex formed in situ from [Ir(OMe)(cod)]2/2(C6F5)3P in the presence of 2-norbornene.

 Please wait while we load your content...
Please wait while we load your content...